Our Pipeline
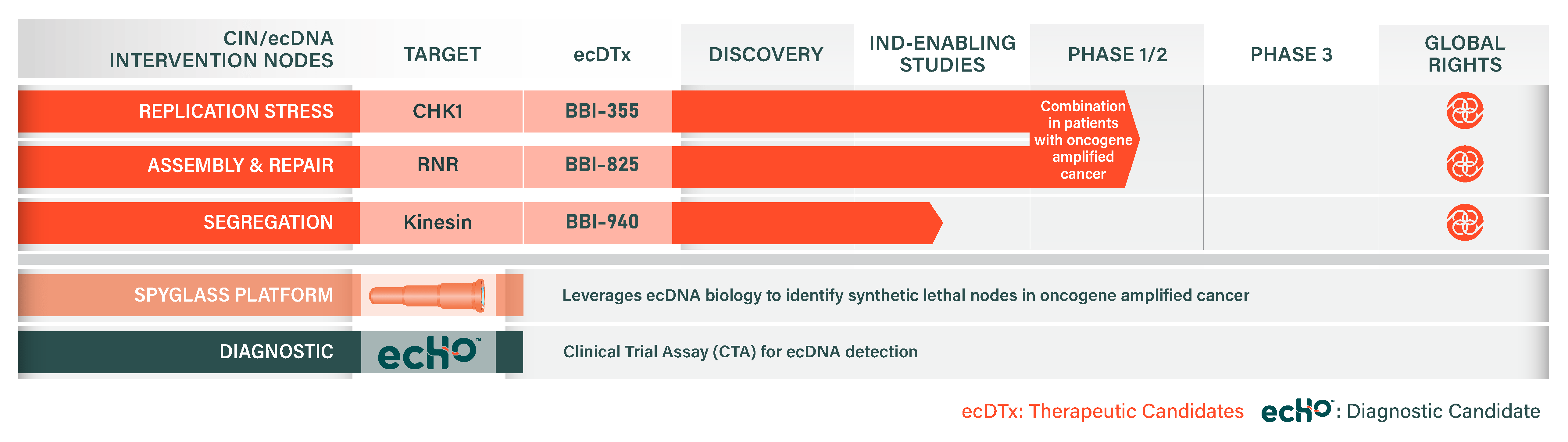
Through our proprietary Spyglass platform and focus on ecDNA biology, we have identified three distinct vulnerabilities of oncogene amplified cancers: (1) Replication Stress, (2) DNA Assembly and Repair, and (3) DNA Segregation.
Replication Stress: BBI-355
Cancer cells with oncogene amplifications, including on ecDNA, exhibit elevated levels of replication stress (RS), a cellular condition where the DNA replication process is dysregulated and leads to potentially toxic DNA damage to cells. As a consequence, tumor cells with high copy number gene amplifications undergoing elevated RS invoke certain cellular RS response factors, exposing a synthetic lethal vulnerability, which renders them hypersensitive to inhibition of such factors.
Checkpoint kinase 1 (CHK1) is one of cancer cells’ master regulators of the DNA Damage Response to RS. BBI-355 is a novel, oral, potent, and selective inhibitor of CHK1. BBI-355 is designed to exploit the elevated RS in ecDNA-enabled oncogene amplified cancer cells by disrupting proper CHK1 function in regulating RS and thereby facilitating mitotic catastrophe to preferentially kill cancer cells relative to healthy cells. The preclinical and clinical development of BBI-355 was designed with the aim to effectively treat oncogene amplified cancers and overcome shortcomings of prior CHK1 inhibitors
DNA Assembly and Repair: BBI-825
In order to maintain and perpetuate oncogene amplifications, including those on ecDNA, gene amplified cancer cells have increased demand for the raw materials used for DNA synthesis and repair. As a consequence, disruption of the supply of raw materials necessary for DNA assembly and repair can sensitize oncogene amplified cancer cells, including those that rely on ecDNA.
Ribonucleotide reductase (RNR) is the rate-limiting enzyme essential for de novo synthesis of deoxyribonucleotide triphosphates (dNTPs), the building blocks of DNA required for its assembly and repair. Inhibition of RNR increases RS and is synthetically lethal to cancer cells that rely on gene amplification for survival, such as those leveraging ecDNA amplification as a primary driver or as a resistance mechanism to overcome treatment.
BBI-355 / BBI-825: novel replication stress combination
In addition to synergizing with certain targeted therapies in oncogene-amplification mediated resistance, inhibition of RNR potentiates the cytotoxicity of CHK1 inhibition in high-RS tumor cells. Since high gene amplification and ecDNA-driven cancer cells have elevated RS, treatment with RNR inhibitors, like BBI-825, exacerbates RS and further increases reliance on CHK1 for survival. By simultaneously inactivating CHK1 and RNR, tumor cells are forced into replication and mitotic catastrophe, leading to cancer cell death.
In combination, BBI-355 and BBI-825 demonstrated evidence of synergistic cytotoxicity in cancer cell lines and tumor regression in mouse xenograft models using weekly dosing. Unlike non-selective, nucleoside analog RNR inhibitors such as gemcitabine, the selective RNR inhibitor BBI-825 is non-myelosuppressive and may have lower risk of hematological toxicity in combination with BBI-355. Boundless plans to initiate clinical development of the BBI-355 + BBI-825 combination in 2025.
DNA Segregation: BBI-940
ecDNA lack centromeres, the structural component of a chromosome that is required for proper segregation of chromosomes during cell division. Thus, segregation and subsequent inheritance of ecDNA in cancer cells is dependent on alternative mitotic mechanisms. We have identified a novel kinesin target that appears to be critical to the segregation of ecDNA in cancer cells but is non-essential in healthy cells. Inhibition of this Kinesin reduced ecDNA and showed synthetic lethality and anti-tumor activity in multiple cancer models.
BBI-940 is a novel, oral, potent, selective degrader of this kinesin, which has demonstrated robust anti-tumor activity in a range of cancer cell lines as well as single agent tumor regressions in mouse xenograft cancer models. Boundless plans to submit an investigational new drug application for BBI-940 in the first half of 2026.
New ecDNA Targets and Vulnerabilities
We have used our proprietary Spyglass platform (see description below) leveraging ecDNA biology to identify and preclinically validate additional therapeutic targets for oncogene amplified cancers. These candidate targets span multiple, diverse synthetic lethal nodes in oncogene amplified cancers and could form the basis for future ecDTx discovery efforts.
Spyglass: the only platform built to identify vulnerabilities of ecDNA in cancer
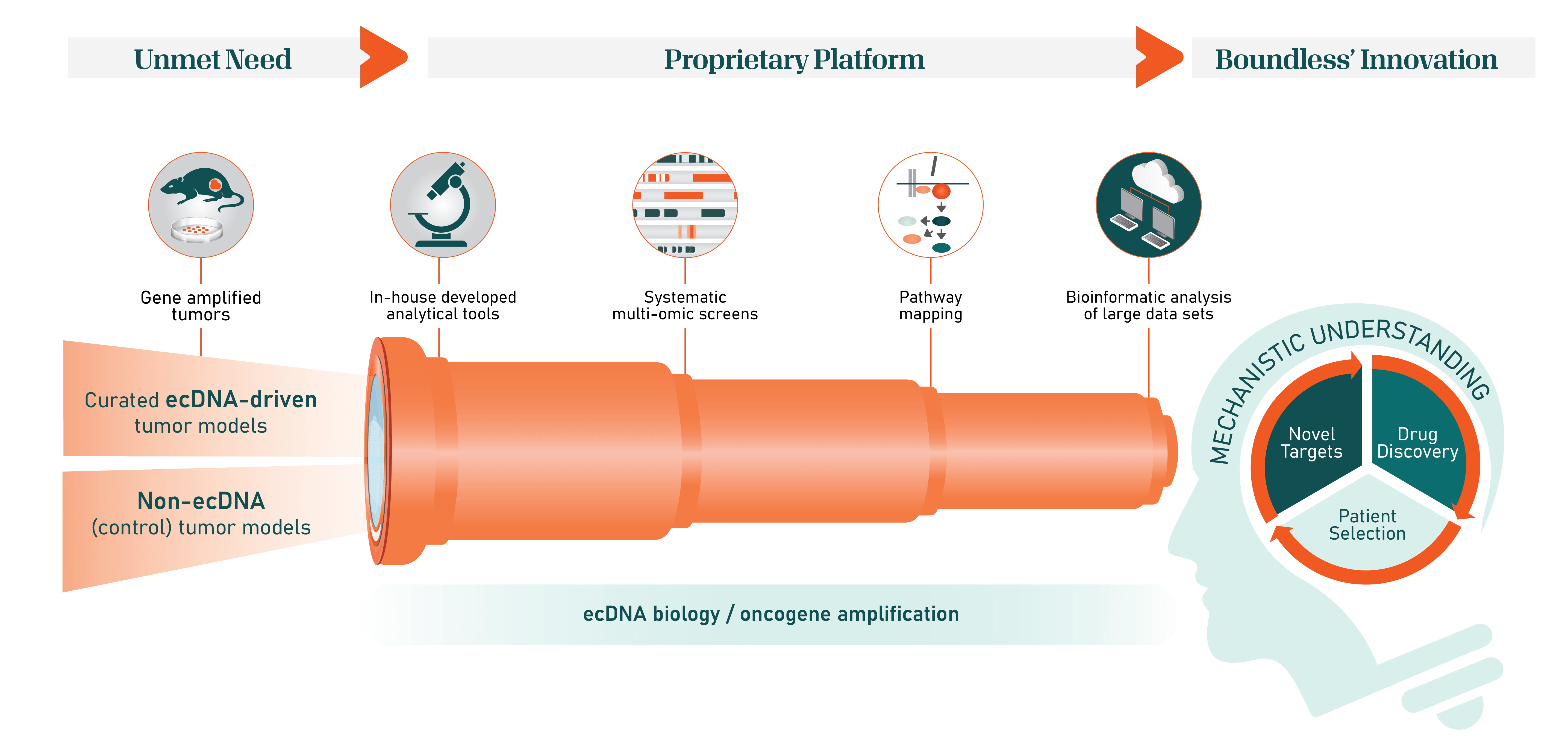
The Spyglass platform consists of a comprehensive suite of proprietary ecDNA+/- models that span many tumor types and amplified oncogenes. Leveraging innovative molecular analytical technologies and imaging techniques, Spyglass enables characterization of ecDNA in cancer cells and reveals a synthetic lethality-based approach to targeting ecDNA-enabled cancers. Through Spyglass, Boundless Bio scientists have thus far identified and validated several distinct and druggable cellular targets that are essential to the function of ecDNA in cancer cells.
ecDNA are the next frontier of precision medicine

ECHO (ecDNA Harboring Oncogenes) is a proprietary software algorithm for detecting the presence of ecDNA using routine clinical next-generation sequencing (NGS) data. ECHO is the first ecDNA diagnostic used in clinical trials.
In our mission to deliver transformative precision therapies to patients with oncogene amplified cancers, ECHO is a vital tool to select appropriate patients for treatment with our ground-breaking ecDTx candidates.
Foundational
Publications
Reviews
Examples of unmet need in
oncogene amplified cancer
Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer
COHEN 2017 ANNALS OF ONCOLOGY
ReadFutibatinib, an Irreversible FGFR1-4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/ FGFR Aberrations: A Phase I Dose-Expansion Study
MERIC-BERNSTAM 2022 CANCER DISCOVERY
ReadProgression-Free Survival Among Patients With Well-Differentiated or Dedifferentiated Liposarcoma Treated With CDK4 Inhibitor Palbociclib: A Phase 2 Clinical Trial
DICKSON 2016 JAMA ONCOLOGY
ReadThe rapidly growing field of ecDNA cancer biology helps explain why some oncogene amplified cancers are so aggressive and why traditional approaches to treatment are not working for many patients. This new understanding of ecDNA shines light onto innovative and differentiated therapeutic approaches to help those affected by the most aggressive tumors. I am thrilled that Boundless Bio has assembled a world class team that has been able to leverage ecDNA biology to develop a host of innovative drug candidates that have the chance to improve the lives of patients with oncogene amplified cancer.”
Paul Mischel, M.D.
Scientific Co-Founder and Chairman of the Scientific Advisory Board

